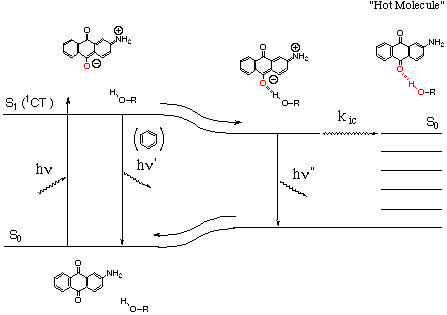
Figure Radiationless deactivation through hydrogen-bonding interaction.
Aminoanthraquinones (AAQ) and aminofluorenones are characteristic molecules that exhibit strong intermolecular hydrogen-bonding interaction in the excited state, since the excited states have a strong intramolecular charge-transfer nature. The large electron density on the carbonyl oxygen of AAQ in the excited state strongly promotes an intermolecular hydrogen-bonding interaction with nonconjugated molecule such as an alcohol. We have reported an efficient deactivation of the excited singlet state of AAQ in ethanol. An intermolecular hydrogen-bonding with the hydroxyl group of alcohol was revealed to be a dominant mode of radiationless deactivation to the ground state, and the electronic excited energy was supposed to dissipate through the hydrogen bond as vibrational energy.

Figure Radiationless deactivation through hydrogen-bonding
interaction.
Recently, we have found that not only alcohol but also hydroperoxide quenched the fluorescence of AAQ. With the fluorescence-quenching the decomposition of hydroperoxide was observed. The decomposition of hydroperoxide could mean that dissipated energy through the intermolecular hydrogen bond was converted selectively to induce chemical reaction such as bond cleavage. Stern-Volmer analyses for the obtained products show that the decomposition was closely related to fluorescence-quenching process. From the viewpoints of energy dissipation, upon radiationless deactivation from the electronically excited state the excited energy has generally been accepted to dissipate randomly through the surrounding solvent molecules. The sensitized decomposition of hydroperoxide coupled with radiationless deactivation strongly suggests that the excited energy is not randomly but at least partly selectively transferred to a specific molecule surrounding the chromophore in the case. An investigation of the microscopic molecular mechanism of radiationless deactivation by intermolecular hydrogen-bonding interactions would clarify the problem of whether the energy dissipation is inherently isotropic or anisotropic.
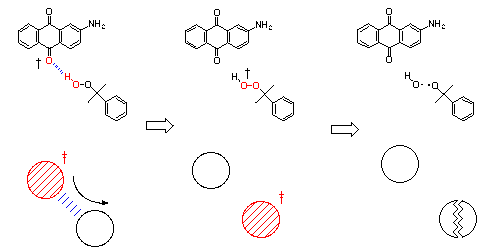
Figure Decomositon of hydroperoxide coupled with radiationless
deactivation.