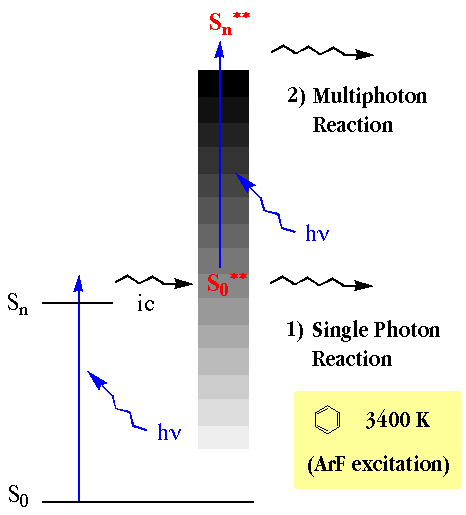
- The high temperature conditions can be created by photoirradiation. The highly vibrationally excited state, namely a hot molecule, which has an equivalent vibrational temperature of 2000-4000 K, would be produced after a rapid internal conversion from an electronically excited state. The hot molecules are known to be formed by the VUV-UV laser irradiation of gaseous molecules. However, studies of the hot molecule reactions using lasers were limited to a small variety of molecules. The molecules other than aromatic hydrocarbons and olefins have not been well studied. The large molecules such as aromatic hydrocarbons larger than naphthalene have also not been well studied because they did not have a sufficient vapor pressure at ambient temperature. We have reported the hot molecule chemistry of large molecules such as naphthalene and 2, 2-paracyclophane.The large molecules, which have a low vapor pressure at room temperature, could be used for the experiments by elevating the experimental temperature for efficient vaporization and for enough absorbance at the laser wavelength. In the case of 2,2-paracyclophane, the experiments were carried out at 453 K. In these cases, product formations by the two-photon process were observed.